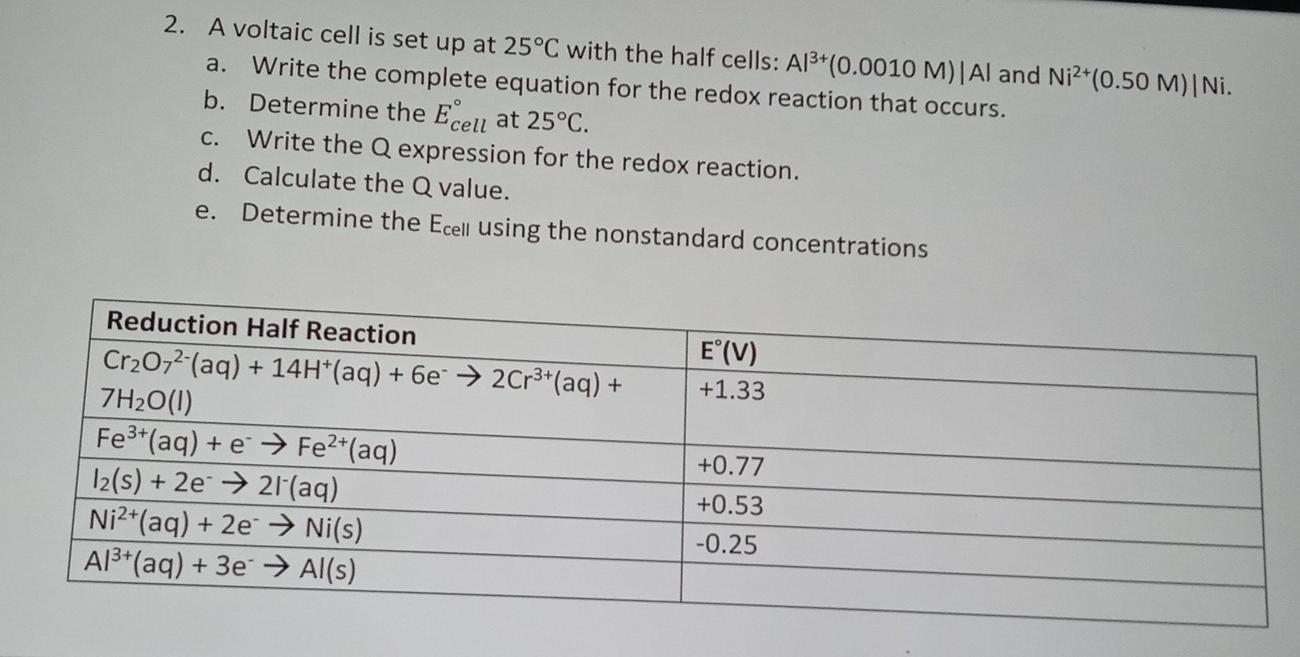
A voltaic cell is set up 25° C with the following half-cells: - Sarthaks eConnect | Largest Online Education Community
A voltaic cell is set up 25° C with the following half-cells: - Sarthaks eConnect | Largest Online Education Community
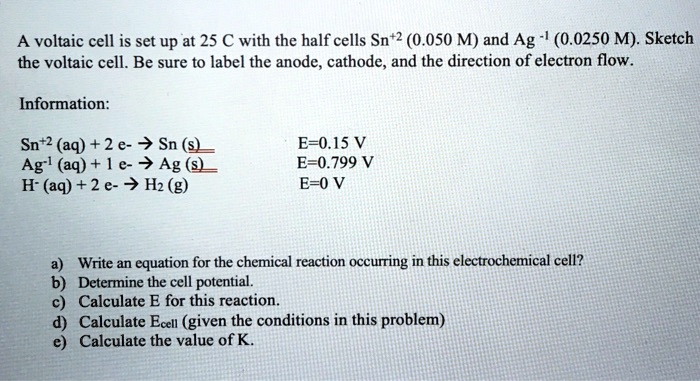
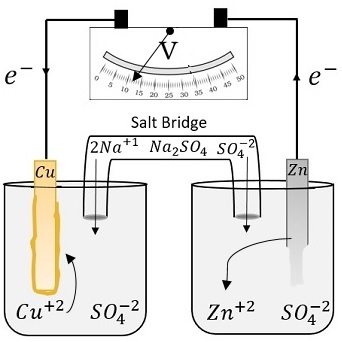
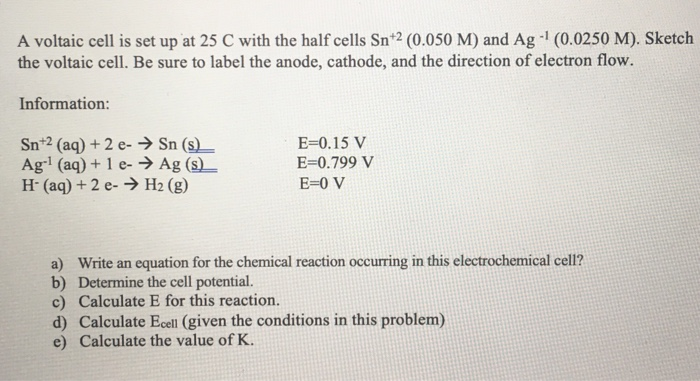
SOLVED: A voltaic cell is set up at 25 C with the half cells Sn+2 (0.050 M) and Ag (0.0250 M). Sketch the voltaic cell. Be sure t0 label the anode, cathode
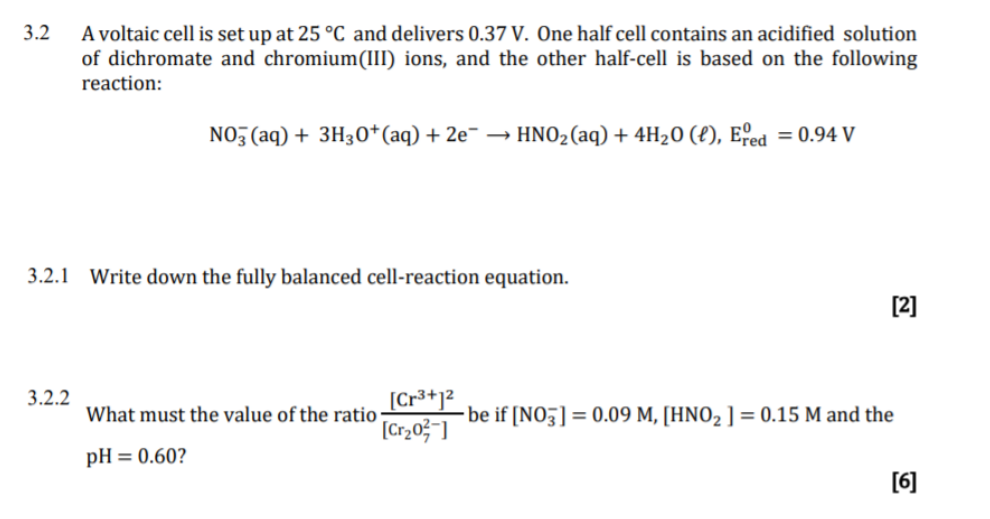
Please answer this Q1 A voltaic cell is set up at 25 C With following 12 - Chemistry - Electrochemistry - 13799617 | Meritnation.com
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not know.the cell potential when measured was 0.422V determine the concentration of

A voltaic cell in set up at 25^oC with the following half cells: Al/Al^3 + (0.001 M) and Ni/Ni^2 + (0.50 M) Write an equation for the reaction that occurs when the

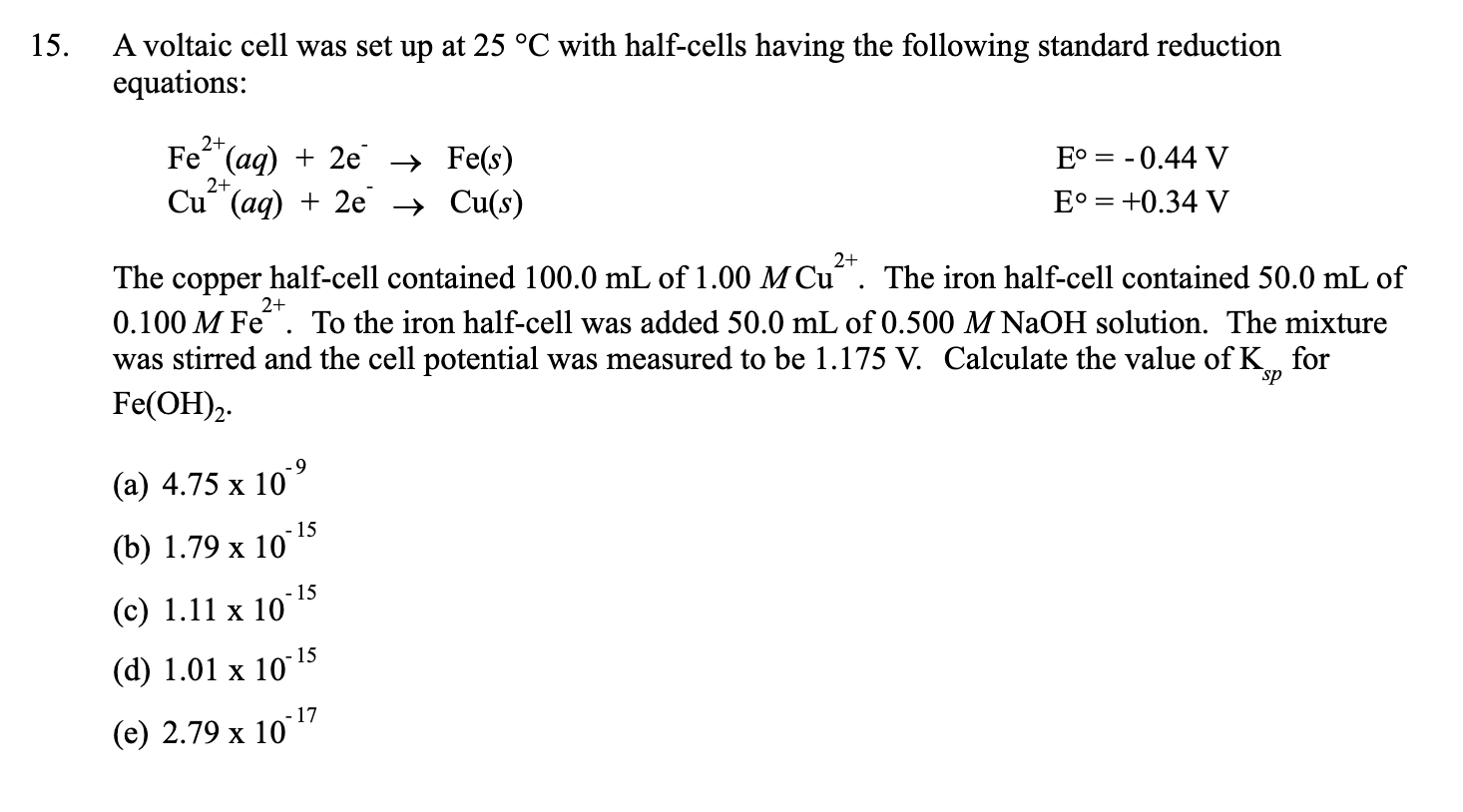
pls answer the whole question 14 A voltaic cell is set up at 25 C with the following - Chemistry - Electrochemistry - 13701485 | Meritnation.com
A voltaic cell is setup at 25°C with the half cells Ag^+ (0.001 M) Ag and Cu^2+ (0.10 M) Cu. What should be its cell potential ? - Sarthaks eConnect | Largest Online Education Community

A voltaic cell in set up at 25^oC with the following half cells: Al/Al^3 + (0.001 M) and Ni/Ni^2 + (0.50 M) Write an equation for the reaction that occurs when the

✓ Solved: A silver concentration cell is set up at 25^°C as shown below: The AgCl(s) is in excess in...
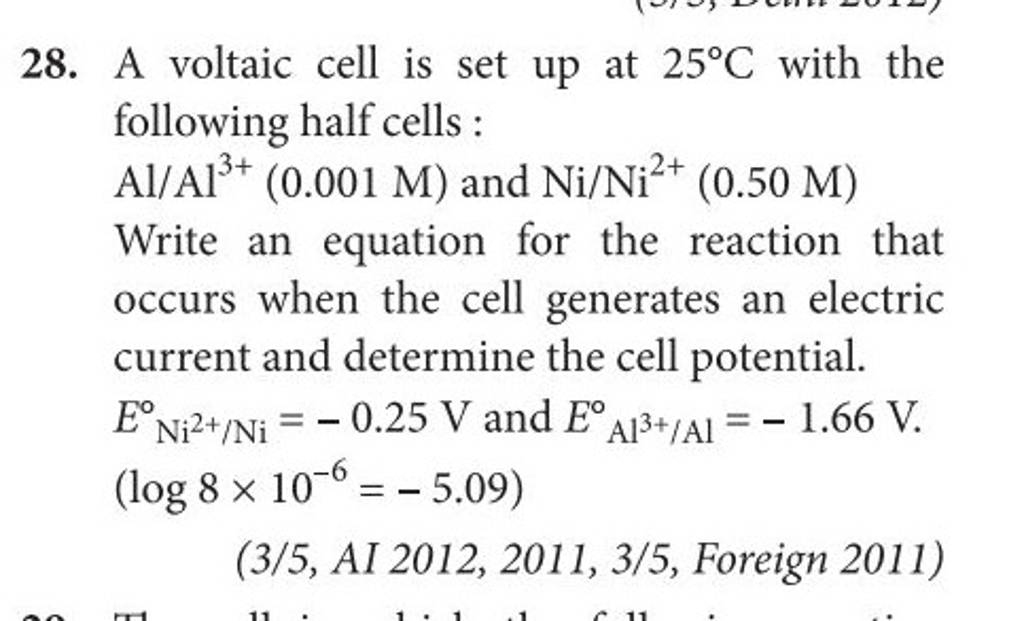
A voltaic cell is set up at 25°C with the following half cells. Al | Al^3+ (0.0010 M) and Ni^2+ (0.50 M) | Ni - Sarthaks eConnect | Largest Online Education Community

taic cell is up 25 °C with the following half cells, 9. 20. A voltaic cell is ur A bovlofti eroinsta A13+ and NiNi2+ (0.001 M) (0.5 M) EX13+ / Al = -

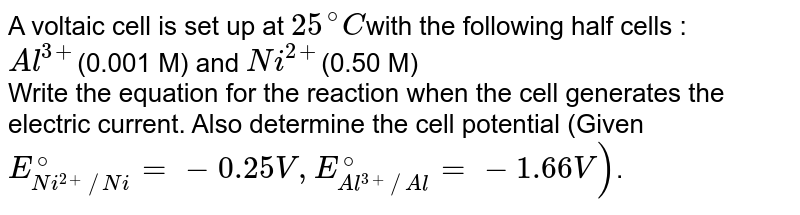

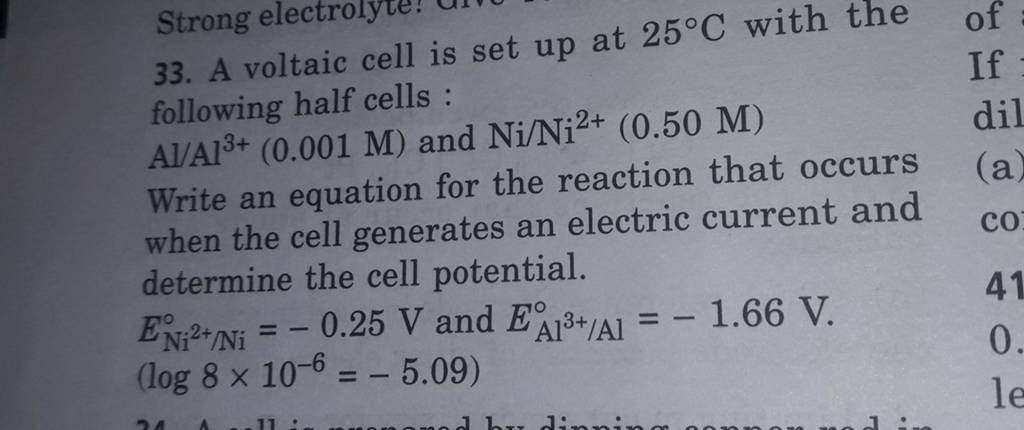


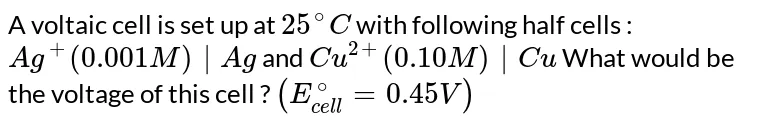


![Kannada] A voltic cell is set up at 25^@C with the half cells Ag^(+) Kannada] A voltic cell is set up at 25^@C with the half cells Ag^(+)](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/7681973.webp)